Teacher Explains
✨ Everyday Fuels
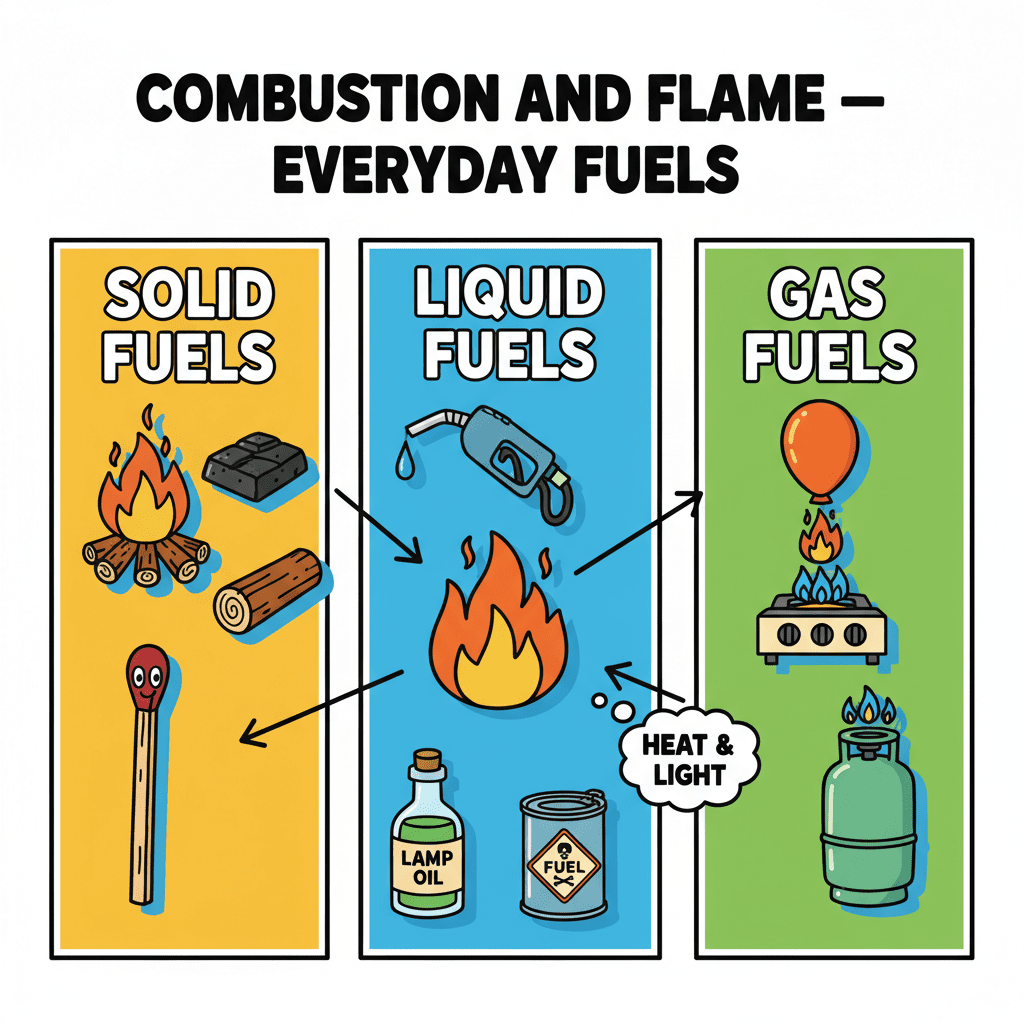
Alright everyone, let's dive into the fascinating world of combustion and flame! Think about all the different kinds of fuels we use every day—at home for cooking, in factories to power machines, and in our cars to get us from place to place. What are some examples that come to mind? Maybe wood, petrol, or even CNG?
✨ Combustion Defined
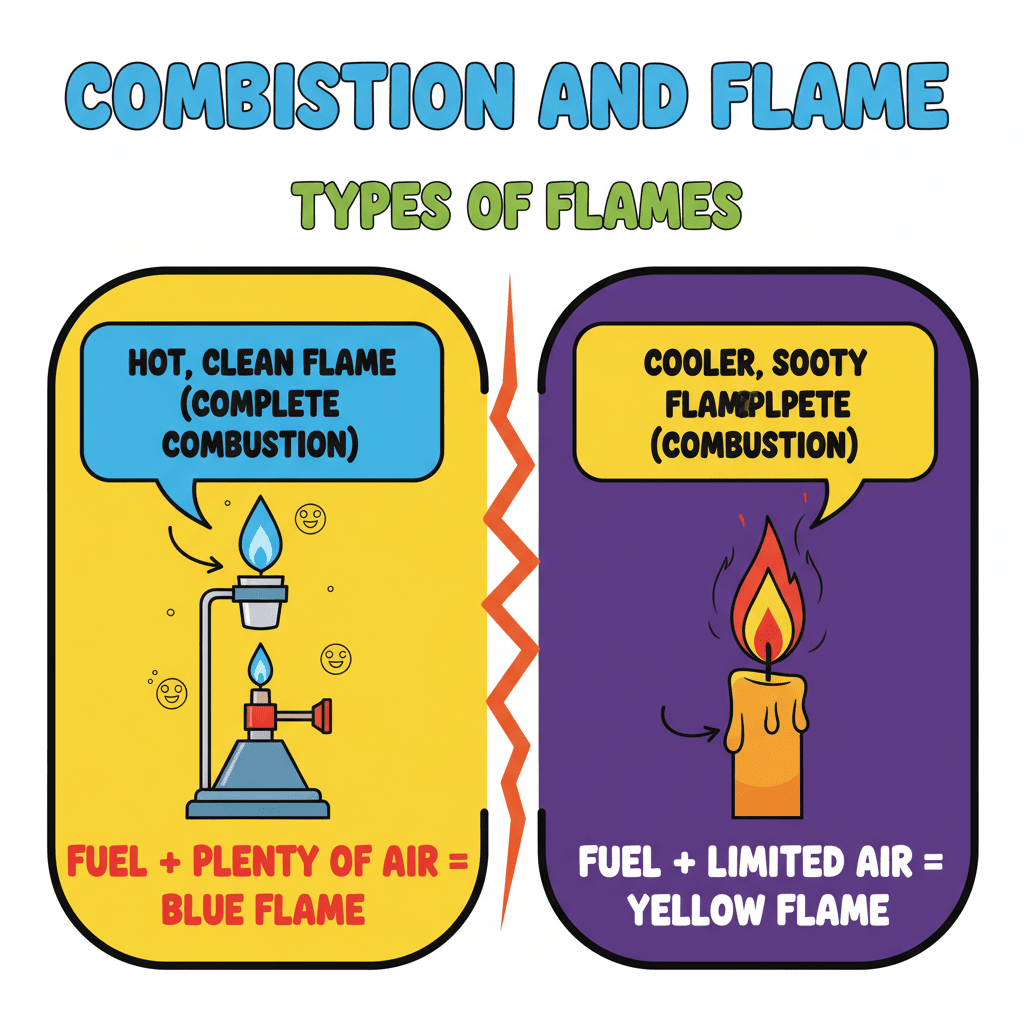
Now, have you ever noticed the difference between burning a candle and burning a piece of coal? A candle has a flame, but coal doesn't! Today, we're going to explore the chemical process of burning, which is called combustion, and take a closer look at the different types of flames that can be produced.
✨ Combustible Substances
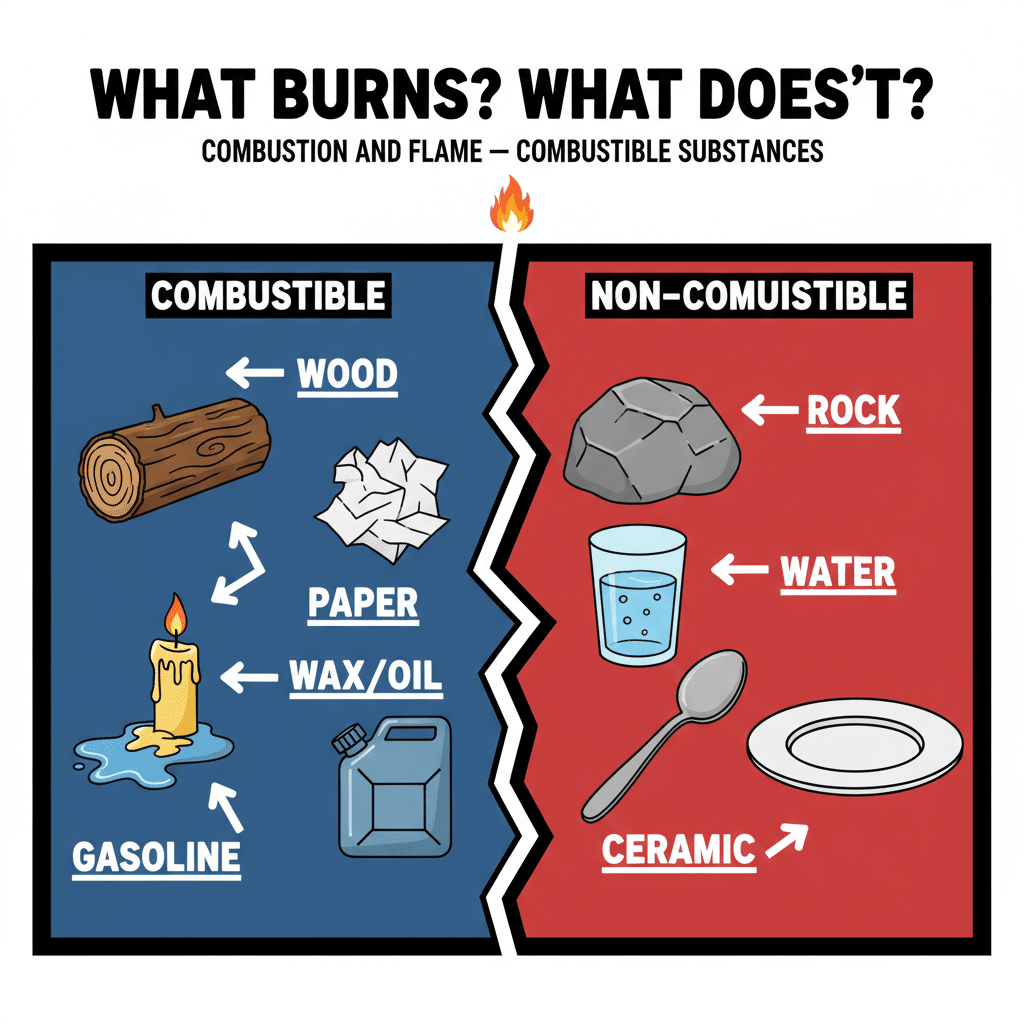
Remember that magnesium ribbon experiment we did last year? When magnesium burns, it creates magnesium oxide, along with heat and light. Similarly, if you hold a piece of charcoal near a flame, you'll see it burns in the air. Coal also burns in air, producing carbon dioxide, heat, and light. So, what exactly is combustion?
✨ Types of Flames
Combustion is a chemical process where a substance reacts with oxygen to produce heat. That substance, the one doing the burning, is called combustible, or sometimes just a fuel. Fuels can be solids, liquids, or gases. And sometimes, when things burn, they produce light, either as a flame or a glow. So, in those earlier examples, magnesium and charcoal are both combustible substances. Even food is a fuel for our bodies!
